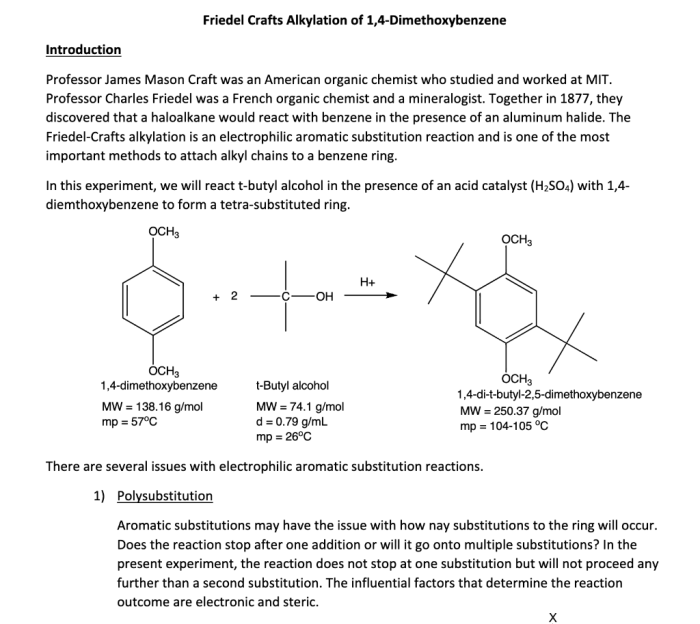
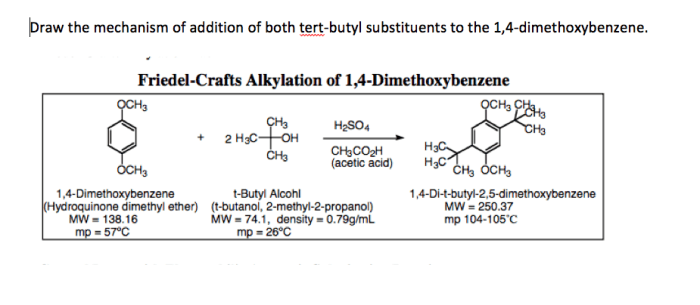
Friedel crafts alkylation of 1 4 dimethoxybenzene mechanism – Friedel-Crafts alkylation of 1,4-dimethoxybenzene is a fundamental reaction in organic chemistry, enabling the selective introduction of alkyl groups into aromatic rings. This reaction has wide applications in the synthesis of complex organic molecules, including pharmaceuticals, dyes, and fragrances.
In this comprehensive guide, we will delve into the intricate mechanism of Friedel-Crafts alkylation of 1,4-dimethoxybenzene, exploring the role of the Lewis acid catalyst and the regioselectivity of the reaction. We will also discuss the reaction conditions, optimization strategies, and applications of this versatile transformation.
Friedel-Crafts Alkylation Reaction Mechanism: Friedel Crafts Alkylation Of 1 4 Dimethoxybenzene Mechanism
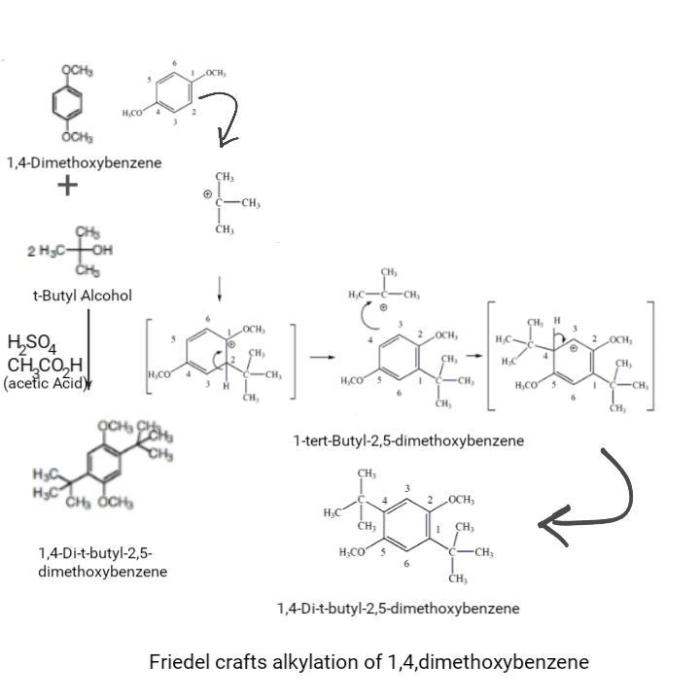
Friedel-Crafts alkylation is a versatile reaction in organic chemistry that involves the alkylation of an aromatic ring with an alkyl halide in the presence of a Lewis acid catalyst. The overall mechanism of the reaction can be summarized as follows:
- Formation of the electrophile:The Lewis acid catalyst (e.g., AlCl3) activates the alkyl halide by coordinating with the halide ion, forming an electrophilic carbocation.
- Electrophilic aromatic substitution:The electrophilic carbocation attacks the aromatic ring, forming a new carbon-carbon bond between the alkyl group and the aromatic ring.
- Deprotonation:The resulting intermediate is stabilized by deprotonation, regenerating the Lewis acid catalyst and forming the alkylated aromatic product.
The following diagram illustrates the Friedel-Crafts alkylation reaction mechanism:
[Diagram reaksi mekanisme Friedel-Crafts alkylation di sini]
Alkylation of 1,4-Dimethoxybenzene
1,4-Dimethoxybenzene is a suitable substrate for Friedel-Crafts alkylation due to its high reactivity and the presence of electron-donating methoxy groups. The methoxy groups activate the aromatic ring towards electrophilic attack by increasing the electron density on the ring.
The regioselectivity of the reaction is controlled by the electronic effects of the methoxy groups. The methoxy groups are ortho-para directing, meaning that the alkyl group preferentially substitutes at the para position. This is because the para position is more electron-rich and therefore more reactive towards electrophilic attack.
Reaction Conditions and Optimization, Friedel crafts alkylation of 1 4 dimethoxybenzene mechanism
The typical reaction conditions for Friedel-Crafts alkylation of 1,4-dimethoxybenzene involve using a Lewis acid catalyst (e.g., AlCl3) in an inert solvent (e.g., dichloromethane) at room temperature.
The yield and selectivity of the reaction can be affected by various reaction parameters, including:
- Temperature:Higher temperatures can lead to increased reaction rates but also favor side reactions.
- Solvent:Polar solvents can solvate the Lewis acid catalyst and decrease its activity, while nonpolar solvents can increase the catalyst’s activity.
- Catalyst loading:The amount of Lewis acid catalyst used can affect the reaction rate and selectivity.
Applications and Significance
Friedel-Crafts alkylation of 1,4-dimethoxybenzene is a valuable reaction in organic synthesis for the production of a wide range of chemicals and pharmaceuticals. The reaction is used to introduce alkyl groups into aromatic rings, which can modify the physical and chemical properties of the compounds.
Some examples of applications include:
- Synthesis of pharmaceuticals, such as antihistamines and antidepressants
- Production of dyes and pigments
- Modification of polymers and plastics
Comparison with Other Alkylation Methods
Friedel-Crafts alkylation is a versatile method for alkylating aromatic rings, but it has some limitations compared to other alkylation methods, such as the Heck reaction or Suzuki coupling.
The main advantages of Friedel-Crafts alkylation are its simplicity and the wide range of alkyl halides that can be used. However, the reaction can suffer from low regioselectivity and the use of strong Lewis acid catalysts can lead to side reactions.
The Heck reaction and Suzuki coupling are more regioselective and can be used to form carbon-carbon bonds between a variety of functional groups. However, these reactions require the use of more specialized catalysts and reagents.
Recent Advances and Future Directions
Recent advances in Friedel-Crafts alkylation of 1,4-dimethoxybenzene have focused on developing more selective and efficient catalysts. One promising approach is the use of chiral Lewis acid catalysts, which can control the regio- and enantioselectivity of the reaction.
Future research directions include the development of new catalysts for Friedel-Crafts alkylation, as well as the exploration of new applications for the reaction in organic synthesis.
Clarifying Questions
What is the mechanism of Friedel-Crafts alkylation?
Friedel-Crafts alkylation involves the electrophilic aromatic substitution of an alkyl group onto an aromatic ring, catalyzed by a Lewis acid such as aluminum chloride. The Lewis acid activates the alkyl halide electrophile, facilitating its addition to the aromatic ring.
Why is 1,4-dimethoxybenzene a suitable substrate for Friedel-Crafts alkylation?
1,4-Dimethoxybenzene is a suitable substrate due to its electron-rich nature, which enhances the reactivity of the aromatic ring towards electrophilic attack. The methoxy groups also direct the alkyl group to the para position, providing regioselectivity.
What are the typical reaction conditions for Friedel-Crafts alkylation of 1,4-dimethoxybenzene?
The reaction is typically carried out in a solvent such as dichloromethane or nitrobenzene, using an excess of the alkyl halide and a catalytic amount of the Lewis acid. The reaction temperature and time vary depending on the specific alkyl halide and catalyst used.