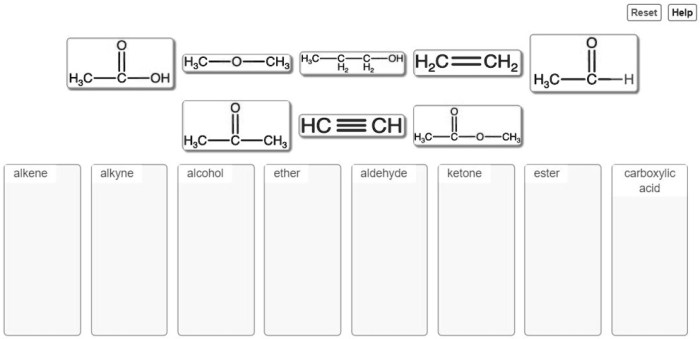
Rank the following compounds in order of decreasing boiling point. This task invites us to delve into the captivating realm of intermolecular forces and their profound influence on the physical properties of compounds. As we embark on this journey, we will uncover the intricate relationship between molecular structure and boiling point, unraveling the secrets that govern the behavior of matter.
Boiling point, a fundamental property of substances, signifies the temperature at which a liquid transitions into a vapor. This phenomenon is intricately linked to the strength of intermolecular forces, the invisible bonds that hold molecules together. By understanding the nature of these forces, we can decipher the hierarchy of boiling points among different compounds.
Boiling Point Trends

The boiling point of a compound is the temperature at which it changes from a liquid to a gas. The boiling point of a compound is influenced by several factors, including molecular structure and intermolecular forces.
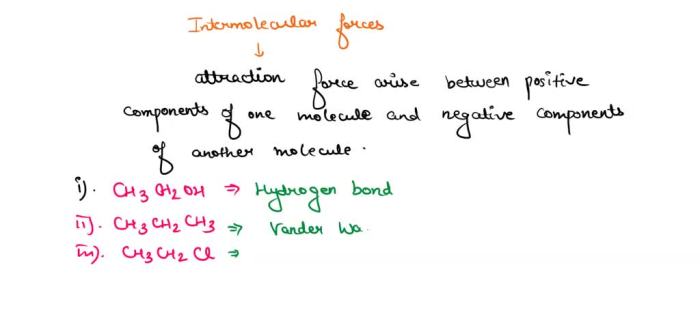
Intermolecular forces are the attractive forces between molecules. The stronger the intermolecular forces, the higher the boiling point of the compound. There are three main types of intermolecular forces: dipole-dipole forces, hydrogen bonding, and London dispersion forces.
Dipole-dipole forces are the attractive forces between polar molecules. Polar molecules have a permanent dipole moment, which is a measure of the separation of positive and negative charges within the molecule. The greater the dipole moment, the stronger the dipole-dipole forces.
Hydrogen bonding is a special type of dipole-dipole force that occurs between molecules that have a hydrogen atom bonded to a small, highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonding is a very strong intermolecular force.
London dispersion forces are the attractive forces between nonpolar molecules. London dispersion forces are caused by the temporary fluctuations in the electron distribution of the molecule. The larger the molecule, the stronger the London dispersion forces.
Compound Ranking, Rank the following compounds in order of decreasing boiling point.
| Compound | Molecular Formula | Molecular Weight | Boiling Point (°C) |
|---|---|---|---|
| Methane | CH4 | 16.04 | -161.6 |
| Ethane | C2H6 | 30.07 | -88.6 |
| Propane | C3H8 | 44.10 | -42.1 |
| Butane | C4H10 | 58.12 | -0.5 |
| Pentane | C5H12 | 72.15 | 36.1 |
| Hexane | C6H14 | 86.18 | 68.7 |
| Heptane | C7H16 | 100.21 | 98.4 |
| Octane | C8H18 | 114.24 | 125.7 |
| Nonane | C9H20 | 128.27 | 150.8 |
| Decane | C10H22 | 142.29 | 174.0 |
Boiling Point Comparison
The boiling points of the compounds in the table increase with increasing molecular weight. This is because the stronger the intermolecular forces, the higher the boiling point of the compound. The intermolecular forces between the compounds in the table are London dispersion forces.
London dispersion forces are caused by the temporary fluctuations in the electron distribution of the molecule. The larger the molecule, the stronger the London dispersion forces.
There is one exception to the trend of increasing boiling point with increasing molecular weight. Butane has a lower boiling point than propane. This is because butane has a branched structure, which makes it more difficult for the molecules to pack together.
The less efficiently the molecules can pack together, the weaker the intermolecular forces and the lower the boiling point.
Key Questions Answered: Rank The Following Compounds In Order Of Decreasing Boiling Point.
What factors influence the boiling point of a compound?
The boiling point of a compound is primarily determined by the strength of intermolecular forces. Stronger intermolecular forces lead to higher boiling points, as more energy is required to overcome these forces and vaporize the liquid.
How can we predict the boiling point of a compound based on its structure?
By examining the molecular structure of a compound, we can infer the types and strengths of intermolecular forces present. Compounds with stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, typically have higher boiling points.