Embark on a scientific odyssey with the POGIL Intermolecular Forces Answer Key, a comprehensive guide to unlocking the mysteries of the forces that govern the behavior of matter. Delve into the captivating realm of intermolecular interactions, where subtle forces shape the properties and phenomena we encounter daily.
POGIL (Process Oriented Guided Inquiry Learning) activities provide an engaging and interactive approach to mastering intermolecular forces. Through hands-on experiments and guided discussions, students explore the concepts, identify different types of forces, and witness their impact on the physical world.
Intermolecular Forces: Pogil Intermolecular Forces Answer Key
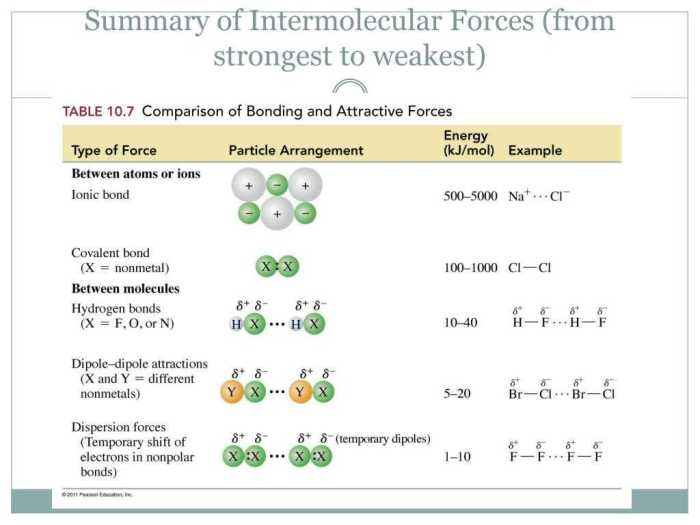
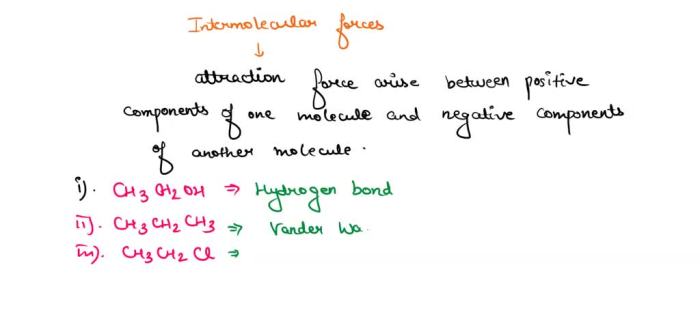
Intermolecular forces (IMFs) are the forces that act between molecules. They are weaker than the intramolecular forces that hold atoms together within a molecule, but they are strong enough to influence the physical properties of matter.
There are three main types of IMFs:
- Dipole-dipole forcesoccur between polar molecules, which have a permanent dipole moment. The positive end of one molecule is attracted to the negative end of another molecule.
- Hydrogen bondingis a special type of dipole-dipole force that occurs between molecules that contain hydrogen atoms bonded to small, highly electronegative atoms such as oxygen, nitrogen, or fluorine.
- London dispersion forcesoccur between all molecules, regardless of their polarity. They are caused by the temporary fluctuations in the electron distribution of a molecule, which creates a temporary dipole moment.
The relative strengths of the three types of IMFs are as follows:
- Hydrogen bonding > dipole-dipole forces > London dispersion forces
POGIL Activities
Process-Oriented Guided Inquiry Learning (POGIL) activities are a type of student-centered learning that emphasizes the development of critical thinking and problem-solving skills. POGIL activities can be used to teach a variety of topics, including intermolecular forces.
POGIL activities typically involve students working in small groups to answer a series of questions. The questions are designed to guide students through the process of inquiry and discovery. POGIL activities can be used to help students develop a deeper understanding of intermolecular forces and their impact on the properties of matter.
Here are some examples of POGIL activities that focus on intermolecular forces:
- IMFs and Boiling Points: This activity helps students understand the relationship between IMFs and the boiling points of liquids.
- IMFs and Phase Transitions: This activity helps students understand the role of IMFs in phase transitions, such as melting, freezing, and vaporization.
- IMFs and Solubility: This activity helps students understand the role of IMFs in solubility.
Answer Key
| Question | Answer |
|---|---|
| 1. What are the three main types of intermolecular forces? | Dipole-dipole forces, hydrogen bonding, and London dispersion forces |
| 2. Which type of IMF is the strongest? | Hydrogen bonding |
| 3. Which type of IMF is the weakest? | London dispersion forces |
| 4. What is the relationship between IMFs and the boiling points of liquids? | The stronger the IMFs, the higher the boiling point |
| 5. What is the role of IMFs in phase transitions? | IMFs hold molecules together in different phases of matter |
| 6. What is the role of IMFs in solubility? | IMFs affect the ability of a solute to dissolve in a solvent |
Applications of Intermolecular Forces, Pogil intermolecular forces answer key
IMFs play an important role in the properties of matter. For example, IMFs are responsible for the following:
- The boiling points of liquids
- The melting points of solids
- The solubility of solids and gases in liquids
- The viscosity of liquids
- The surface tension of liquids
IMFs are also used in a variety of everyday applications, such as:
- Adhesives
- Paints
- Detergents
- Food
- Medicine
IMFs are also important in biological systems. For example, IMFs are responsible for the following:
- The structure of proteins
- The function of enzymes
- The transport of molecules across cell membranes
Top FAQs
What are intermolecular forces?
Intermolecular forces are attractive or repulsive forces that act between molecules, influencing their behavior and properties.
How does the POGIL Intermolecular Forces Answer Key help students?
The answer key provides detailed explanations and guidance for POGIL activities, enabling students to grasp the concepts and apply their knowledge effectively.
What are the different types of intermolecular forces?
Common types include dipole-dipole interactions, hydrogen bonding, and van der Waals forces.